Now that you’ve been prescribed BREO ELLIPTA for your asthma, it’s important to follow your doctor’s instructions and take your medication as directed.
BREO ELLIPTA contains two active ingredients: fluticasone furoate and vilanterol. Fluticasone furoate reduces inflammation (swelling) in the airways while vilanterol works to open and relax the muscles allowing more air to get in and out of the lungs. BREO ELLIPTA does not cure asthma but helps to control it.
You should continue to take BREO ELLIPTA regularly even if you feel fine and do not have any symptoms. Do not stop taking BREO ELLIPTA without talking to your doctor first.
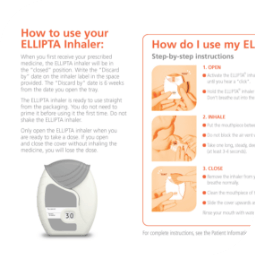
Knowing how to use the ELLIPTA inhaler correctly is also important. Proper technique when using the inhaler device is vital to help ensure that you are taking the medication prescribed to you appropriately.
The resources below will show you how to take your medication properly with the ELLIPTA inhaler. You can also ask your pharmacist to confirm you are using the ELLIPTA correctly when you go in for your refills.
resources for you
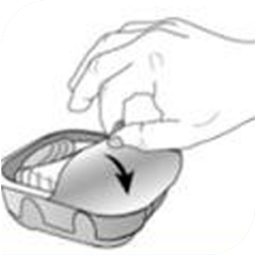
BREO ELLIPTA Patient Information
This is the information leaflet that you will also find inside your BREO ELLIPTA packaging. It provides important information about how to take and store BREO ELLIPTA.
Remember to take your medication as directed, and ensure you are using the ELLIPTA inhaler correctly.
If you need to report an adverse event for any GlaxoSmithKline product, please call: 1-800-387-7374.
BREO ELLIPTA is used for the long-term treatment of asthma in people aged 18 years and older who have asthma that is not adequately controlled with a long-term asthma medication such as an inhaled corticosteroid (ICS) alone; or whose asthma severity requires treatment with both an ICS and a long-acting beta2 agonist (LABA).
Do not use BREO ELLIPTA to treat sudden severe symptoms of asthma such as sudden shortness of breath or wheezing. BREO ELLIPTA is not a rescue inhaler and should not be used to give you fast relief from your asthma attack. You must use a rescue inhaler during a sudden asthma attack. Do not use BREO ELLIPTA if you: are under 18 years of age; are allergic to any of the medicinal or nonmedicinal ingredients contained in the product; have a lactose or severe milk protein allergy.
Before using BREO ELLIPTA, talk to a healthcare professional if you: have liver disease, as you may be more likely to experience side effects; have heart problems, irregular heart beat or high blood pressure; are pregnant or planning to become pregnant; are breastfeeding; have ever had thrush or a yeast infection in your mouth; have ever had seizures; have thyroid gland problems or disease; have diabetes or high blood sugar; have eye problems such as glaucoma, cataracts, blurry vision or other changes in vision; have ever had to stop taking another medication for your breathing problems because you were allergic to it or it caused problems; have been taking other corticosteroids by mouth or by inhalation; have an immune system problem; have any allergies to food or drugs; have low levels of potassium in your blood; have ever had herpes simplex of the eye, a history of tuberculosis infections, or any type of viral, bacterial, fungal (yeast), or parasitic infection.
When LABA medicines are used alone without an ICS, they increase the risk of hospitalization and death from asthma problems. BREO ELLIPTA contains both an ICS and LABA. Studies showed that when an ICS and LABA are used together, there is not a significant increased risk in hospitalizations and death from asthma problems.
Side effects of BREO ELLIPTA may include: itchy, runny or blocked nose (nasopharyngitis); infection of the nose or throat, common cold; sore, raised patches in the mouth or throat caused by a yeast infection (candidiasis/thrush); feeling of pressure or pain in the cheeks and forehead (may be signs of inflammation of the sinuses called sinusitis); pain and irritation in the back of the mouth and throat; headache; voice disorders; abdominal pain; flu (influenza); back pain; cough; nausea; high temperature (fever); dizziness; painful joints; hoarseness and voice changes; respiratory tract infection; anxiety; tremor; muscle spasms.